
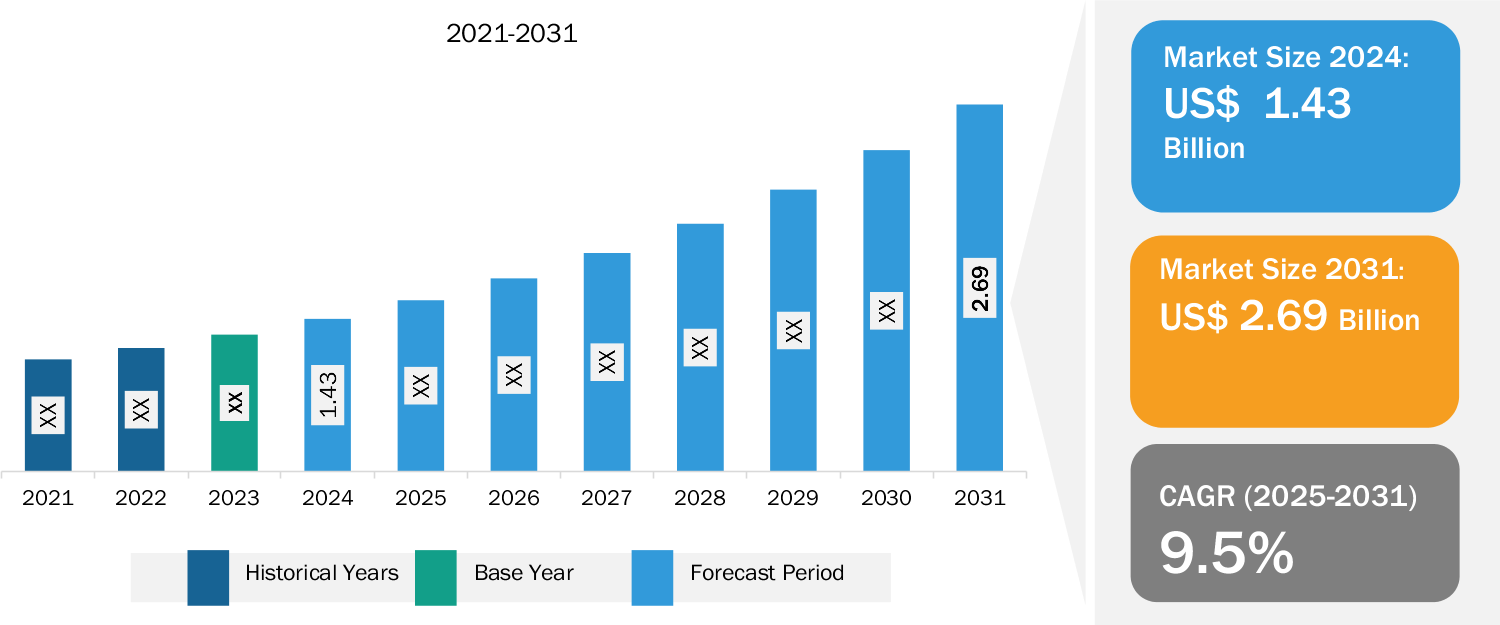
Newborn Screening Market Size to Reach US$ 2.69 billion by 2031, Growing at a CAGR of 9.5%, Says The Insight Partners
The Newborn Screening Market growth is driven by increasing government funding for screening programs, advancements in diagnostic technologies like Next-Generation Sequencing (NGS), and the rising prevalence of congenital disorders. Key players such as LifeCell International Pvt Ltd and PerkinElmer Inc. are leading the market. Governments worldwide are investing in early detection to improve health outcomes for newborns, which further fuels the market’s expansion.
/EIN News/ -- US & Canada, March 24, 2025 (GLOBE NEWSWIRE) -- US & Canada, Mar, 24, 2025 (GlobeNewswire) -- According to a comprehensive report from The Insight Partners, “Newborn Screening Market Size and Forecast, Global and Regional Share, Trend, and Growth Opportunity Analysis Report”, The newborn screening market value is expected to reach US$ 2.69 billion by 2031 from US$ 1.43 billion in 2024; the market is anticipated to register a CAGR of 9.5% during 2025–2031.
To explore the valuable insights in the Newborn Screening Market report, you can easily download a sample PDF of the report- https://www.theinsightpartners.com/sample/TIPHE100001294/
Global Newborn Screening market is observing significant growth owing to the mounting prevalence of CVD and growing cases of osteoporosis bone deformation in adults and newborns.
The report runs an in-depth analysis of market trends, key players, and future opportunities. In general, the Newborn Screening market comprises a vast array of product and services that are expected to register strength during the coming years.
Competitive Strategy and Development
- Key players: LifeCell International Pvt Ltd, Zentech SA, Trivitron Healthcare Pvt Ltd, PerkinElmer Inc, Waters Corp, Bio-Rad Laboratories Inc., Masimo Corp, Natus Medical Inc., Baebies Inc, and MRC Holland BV are among the major companies operating in the newborn screening market.
- Trending topics: Advances in Genetic Testing for Newborns, Expansion of Newborn Screening Panels, Integration of Artificial Intelligence (AI) in Newborn Screening, Integration of Artificial Intelligence (AI) in Newborn Screening, Impact of Expanded Newborn Screening on Public Health, Ethical Considerations in Newborn Screening, Global Variability in Newborn Screening Practices, Implementation of Newborn Screening for Critical Congenital Heart Disease (CCHD).
Headlines on Newborn Screening
- Trivitron Healthcare Launched Two New Tests in the Newborn Screening Panel
- Revvity Launched NGS Panel, Workflow to Complement Current Newborn Screening
- Mylab Introduced a Revolutionary Point-of-Care Newborn Screening Device for Enhanced Healthcare
- Revvity and SCIEX Collaborated to Provide Innovative Neonatal Mass Spectrometry Solution
- Orchard Therapeutics Celebrated Global Progress toward Advancing Newborn Screening for MLD on International Neonatal Screening Day
- Trivitron Healthcare launched #EkSahiShuruat - An Initiative to Raise Awareness on Newborn Screening in India
For Detailed Newborn Screening Market Insights, Visit: https://www.theinsightpartners.com/reports/newborn-screening-market
Newborn Screening Market Overview of Report Findings
1. Market Growth: The newborn screening market value is expected to reach US$ 2.69 billion by 2031 from US$ 1.43 billion in 2024; the market is anticipated to register a CAGR of 9.5% during 2025–2031. The newborn screening market is growing owing to surging government funding for newborn screening and increasing prevalence of newborn disorders. Moreover, the rising burden of congenital diseases is likely to fuel the market expansion in the coming years.
2. Surging Government Funding for Newborn Screening:
Governments globally are recognizing the importance of identifying genetic, metabolic, and congenital disorders in newborns, enabling earlier interventions that can prevent severe health complications later in life.
In the US, the Health Resources and Services Administration (HRSA) has allocated funds to support state-based newborn screening programs, expanding the number of disorders screened. Below are a few instances:
- Newborn screening Co-Propel strengthens the State Newborn Screening Systems Priorities Programs by adding US$ 3.4 million to the US$ 9.3 million awarded in 2023.
- In August 2024, the Australian government invested US$ 5.5 million into a newborn screening platform to screen hundreds of life-threatening genetic illnesses at birth. Funding is from the Medical Research Future Fund (MRFF).
- In August 2024, Emory University’s Medical Nutrition Therapy for Prevention (MNT4P) program was awarded US$2 million to assess and optimize the effectiveness of newborn screening programs in the Southeast US over the next four years.
Similarly, the World Health Organization (WHO) has endorsed universal newborn screening, urging countries to expand screening capabilities. Governments are also funding research to advance screening technologies, such as Next-Generation Sequencing (NGS), to detect a broader range of genetic conditions.
Countries in Europe have made investments in extensive screening systems that incorporate rare disease testing. Globally, these initiatives are guaranteeing improved outcomes for babies and expanding access to early therapies. Therefore, government funding plays a crucial role in advancing technology and making screening programs more accessible.
3. Rising Burden of Congenital Diseases:
Early identification is essential for averting long-term health issues as the prevalence of genetic, metabolic, and congenital illnesses rises worldwide. Early screening enables timely interventions, improving outcomes for affected newborns and reducing the long-term healthcare costs associated with untreated conditions.
Congenital disorders, such as phenylketonuria (PKU), cystic fibrosis, sickle cell anemia, and spinal muscular atrophy (SMA), are being detected earlier through expanded newborn screening programs. For instance, there is no national congenital disabilities tracking system in the US. The majority of states have procedures in place to track certain birth abnormalities. The prevalence of birth abnormalities in the US has been estimated using state data. The table below presents current national estimates of certain birth defects:
|
Specific defects |
How often do they occur? |
How many babies are affected each year? |
| Anencephaly | 1 in 5,246 births |
700 |
| Encephalocele | 1 in 10,365 births | 354 |
| Spina bifida without anencephaly | 1 in 2,875 births | 1,278 |
| Anophthalmia/ microphthalmia |
1 in 5,078 births | 723 |
| Atrioventricular septal defect (Endocardial cushion defect) | 1 in 1,712 births |
2,145 |
After studies demonstrated that early gene therapy treatment might greatly increase survival rates and quality of life, SMA was added to state screening panels in the US. In order to detect these abnormalities early and provide access to treatments that can reduce developmental problems and impairments, governments are placing a greater emphasis on newborn screening.
The World Health Organization (WHO) has highlighted the rising burden of congenital conditions globally, emphasizing the need for expanded newborn screening to address these challenges. In regions with higher birth defects, governments are investing in programs to detect critical congenital heart disease (CCHD) and metabolic disorders to ensure earlier interventions and better patient outcomes. The growing burden of congenital diseases underscores the need for widespread, comprehensive newborn screening programs, driving the market for these diagnostic technologies.
Geographical Insights: In 2023, North America led the market with a substantial revenue share, followed by Europe and Asia Pacific. Asia Pacific is expected to register the highest CAGR during the forecast period.
Stay Updated on The Latest Newborn Screening Market Trends: https://www.theinsightpartners.com/sample/TIPHE100001294/
Market Segmentation
- Based on product type, the newborn screening market is bifurcated into reagents & assay kits and instruments. The reagents & assay kits segment held a larger share of the market in 2024.
- In terms of technology, the newborn screening market is categorized into tandem mass spectrometry (TMS), molecular assays, immunoassays and enzymatic assays, pulse oximetry screening technology, and other technologies. The pulse oximetry screening technology segment dominated the market in 2024.
- Based on test type, the newborn screening market is segmented into dry blood spot tests, hearing screen tests, critical congenital heart disease tests, and other tests. The blood spot test segment dominated the market in 2024.
- By end user, the newborn screening market is segmented into hospitals & clinics and diagnostic laboratories. The hospitals & clinics segment dominated the market in 2024.
- The newborn screening market is segmented into five major regions: North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America.
Purchase Premium Copy of Global Newborn Screening Market Size and Growth Report (2023-2031) at: https://www.theinsightpartners.com/buy/TIPHE100001294/
Conclusion
In conclusion, the market for newborn screening is expanding as a result of advancements in diagnostic technologies, rising rates of congenital and genetic disorders, and increasing government support. Key drivers driving market growth include the expansion of screening panels, the incorporation of state-of-the-art technologies such as Next-Generation Sequencing (NGS), and growing awareness of the significance of early detection. In order to improve healthcare outcomes and lower long-term costs, governments worldwide are funding universal screening programs. The market is expected to grow further due to continuous technical developments and easier access to screening, which will benefit both babies and healthcare systems globally.
The report from The Insight Partners provides several stakeholders—including manufacturers of reagents & assay kits and instruments—with valuable insights to successfully navigate this evolving market landscape and unlock new opportunities.
Related Reports-
- Genomics Market Analysis and Forecast by Size, Share, Growth, Trends 2031
- Prenatal and Newborn Genetic Testing Market Forecast & Growth 2031
- Viral Molecular Diagnostics Market Growth, Top Players, and Forecast by 2031
- Neurometabolic Disorders Market Drivers, Trends, and Forecast by 2031
About Us:
The Insight Partners is a one stop industry research provider of actionable intelligence. We help our clients in getting solutions to their research requirements through our syndicated and consulting research services. We specialize in industries such as Semiconductor and Electronics, Aerospace and Defense, Automotive and Transportation, Biotechnology, Healthcare IT, Manufacturing and Construction, Medical Device, Technology, Media and Telecommunications, Chemicals and Materials.
Contact Us:
If you have any queries about this report or if you would like further information, please contact us:
Contact Person: Ankit Mathur
E-mail: ankit.mathur@theinsightpartners.com
Phone: +1-646-491-9876
Press Release- https://www.theinsightpartners.com/pr/newborn-screening-market

Distribution channels: Banking, Finance & Investment Industry, Business & Economy, Media, Advertising & PR, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release


